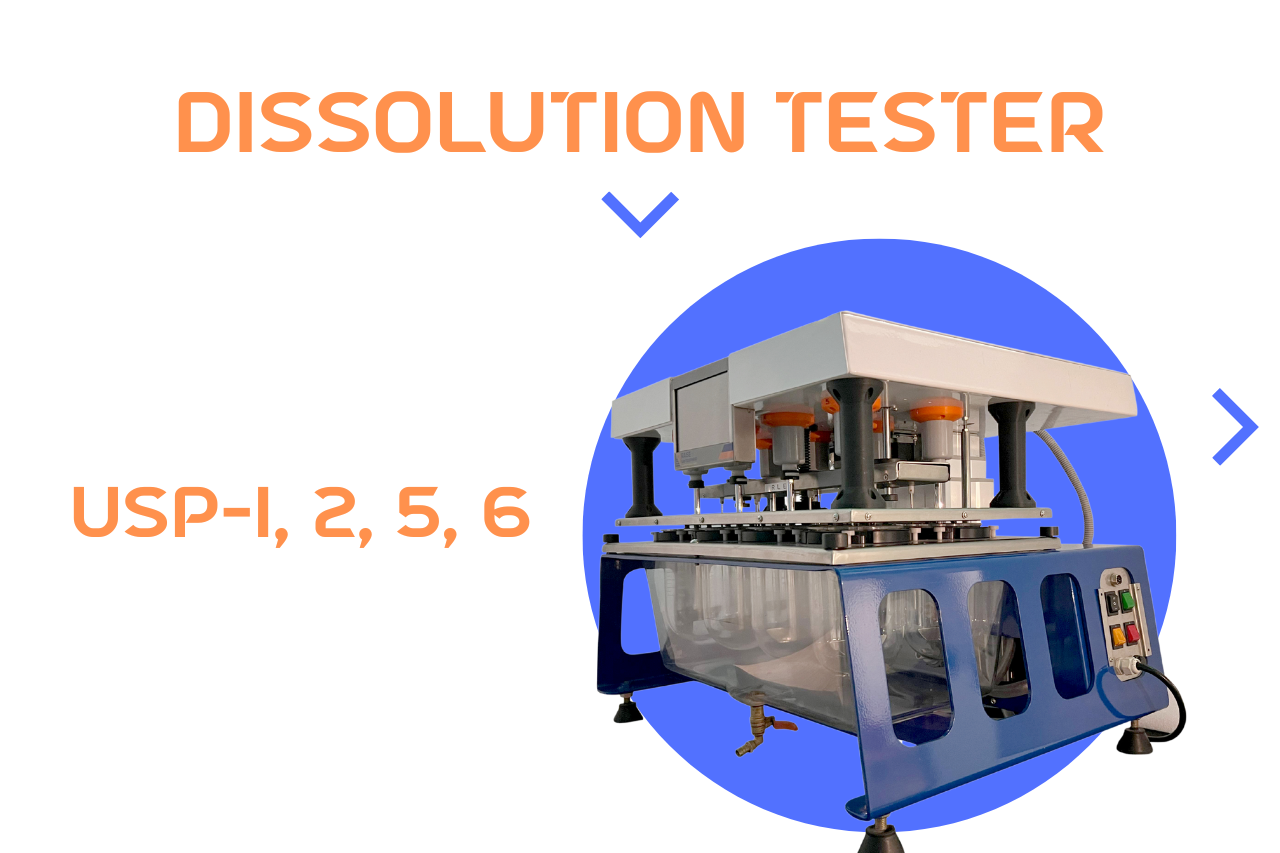
Dissolution Tester USP-1, 2, 5, 6 Methods
Introduction:
Dissolution Tester USP plays a critical role in the pharmaceutical industry by evaluating the rate at which an active pharmaceutical ingredient (API) dissolves in a given medium. It is an essential quality control test used to ensure consistency, efficacy, and bioavailability of oral solid dosage forms such as tablets and capsules. The United States Pharmacopeia (USP) has standardized various dissolution apparatuses, including USP Apparatus 1, 2, 5, and 6, which are widely used in drug development and quality assurance. Additionally, intrinsic dissolution methods provide valuable data regarding the solubility and dissolution characteristics of pure drug substances. This article explores the principles, applications, and distinctions between these dissolution testing methodologies.
USP Dissolution Apparatus
USP Apparatus 1 (Basket Method)
The Basket Method (USP Apparatus 1) is one of the most commonly used dissolution testing techniques. It consists of a cylindrical metal basket attached to a rotating shaft, which holds the dosage form. The basket is immersed in a dissolution medium, and the apparatus maintains controlled temperature and agitation conditions. The rotating motion of the basket helps in exposing the dosage form to the medium, ensuring consistent dissolution.
Applications:
- Suitable for tablets and capsules that tend to float.
- Used for modified-release formulations.
- Ideal for formulations that disintegrate slowly.
USP Apparatus 2 (Paddle Method)
The Paddle Method (USP Apparatus 2) is another widely employed dissolution test. In this setup, a paddle is attached to a rotating shaft and positioned above the dosage form in a dissolution vessel. The paddle’s rotation creates a uniform flow of the medium, allowing the drug to dissolve efficiently.
Applications:
- Preferred for immediate-release tablets and capsules.
- Used for poorly soluble drugs where agitation is necessary.
- Applied in comparative dissolution studies.
USP Apparatus 5 (Paddle over Disk Method)
The Paddle over Disk Method (USP Apparatus 5) is a modification of the paddle method, where the drug formulation is placed on a disk positioned at the bottom of the dissolution vessel. The paddle rotates above the disk, ensuring controlled dissolution.
Applications:
- Primarily used for transdermal patches.
- Suitable for evaluating drug release from semi-solid formulations.
USP Apparatus 6 (Rotating Cylinder Method)
The Rotating Cylinder Method (USP Apparatus 6) is designed for testing the dissolution of transdermal drug delivery systems. It consists of a cylindrical device that rotates in the dissolution medium, ensuring uniform drug release.
Applications:
- Used specifically for transdermal patches.
- Provides insights into the release kinetics of topical and adhesive formulations.
Intrinsic Dissolution Testing
Intrinsic dissolution testing differs from conventional dissolution testing as it focuses on determining the fundamental dissolution rate of a pure drug substance rather than a formulated dosage form. This method helps in understanding the intrinsic solubility and dissolution properties of the API under controlled conditions.
Principle
Intrinsic dissolution testing involves compressing a pure drug substance into a compact disc of defined surface area. The disc is then exposed to the dissolution medium, and the rate at which the drug dissolves is measured. Unlike USP Apparatus 1, 2, 5, or 6, this method provides data that helps in predicting bioavailability and optimizing formulation strategies.
Applications
- Assessing the impact of pH and temperature on drug solubility.
- Predicting dissolution behavior during formulation development.
- Comparing different polymorphic forms of a drug substance.
Comparison of USP Apparatus and Intrinsic Dissolution Testing
| Parameter | USP Apparatus 1 | USP Apparatus 2 | USP Apparatus 5 | USP Apparatus 6 | Intrinsic Dissolution |
| Mechanism | Rotating basket | Rotating paddle | Paddle over disk | Rotating cylinder | Compressed drug disc |
| Application | Tablets, capsules | Tablets, capsule | Transdermal patches | Transdermal patches | Pure drug substance |
| Medium Flow | Moderate | Moderate | Low | Low | Static |
| Drug Release Form | Formulated dosage form | Formulated dosage form | Semi-solid, patches | Semi-solid, patches | API only |
Regulatory Considerations
Dissolution testing is an essential component of regulatory submissions to agencies like the FDA, EMA, and ICH. Pharmaceutical companies must demonstrate that their formulations meet predefined dissolution criteria to ensure batch-to-batch consistency and predict in vivo drug behavior. Regulatory guidelines also recommend conducting biorelevant dissolution testing, simulating physiological conditions to predict actual drug absorption profiles.
Conclusion
Dissolution testing using USP Apparatus 1, 2, 5, and 6, along with intrinsic dissolution methods, plays a pivotal role in the pharmaceutical industry. Each method serves a distinct purpose, ranging from quality control of oral dosage forms to evaluating drug release from transdermal patches and assessing the intrinsic dissolution characteristics of APIs. A comprehensive understanding of these techniques aids in optimizing drug formulations, ensuring regulatory compliance, and ultimately enhancing therapeutic efficacy. By implementing the appropriate dissolution testing methodology, pharmaceutical scientists can develop safer and more effective drug products for patients worldwide.
Visit Here for Calibration and Validation Process: